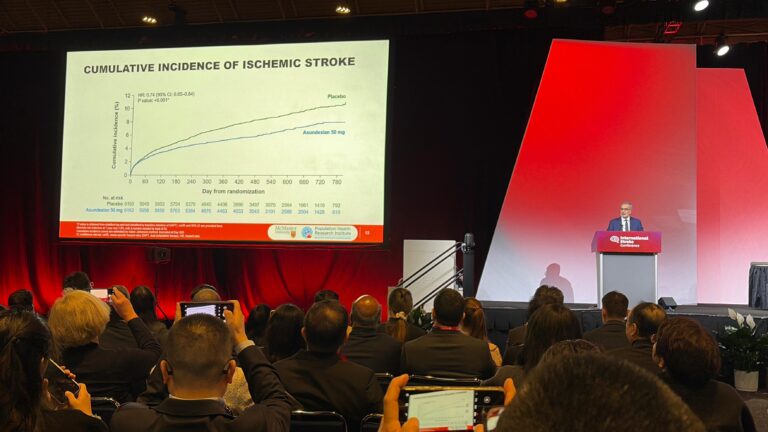
From improving clinical trials across Canada to supporting homegrown biotech, PHRI Deputy Director and Senior Scientist PJ Devereaux highlighted the opportunities and challenges facing the country at the Clinical Trials Ontario 2025 Conference.
In an update on the Accelerating Clinical Trials (ACT) Consortium, for which PHRI serves as the coordinating centre, Devereaux walked attendees through the progress made over the past year to make running clinical trials easier, faster, and more effective. He also underscored the importance of investing in trials that evaluate Canadian-made biotechnologies.
At the panel “Seizing the Canadian Opportunity,” Devereaux and other panelists explored how Canada can strengthen its role in global clinical trials. With new national investments, stronger cross-country collaboration, and ongoing work to reduce interprovincial barriers, panelists discussed how Canada can turn today’s momentum into a long-term advantage for patients, families, and the life sciences sector.