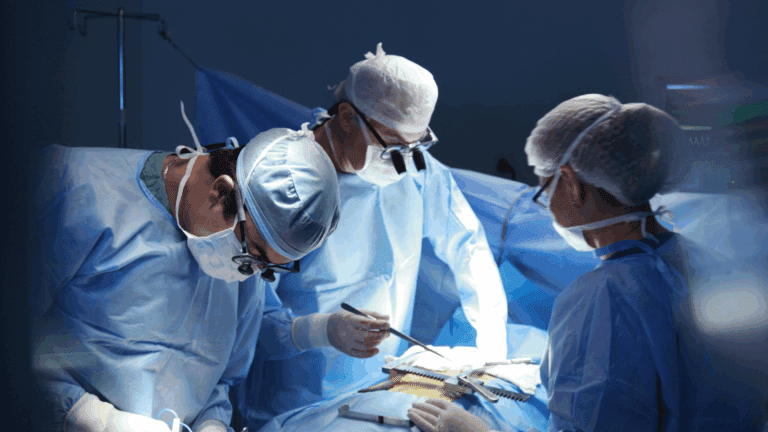
The first participant in a large, global trial led by PHRI has undergone the implantation of a left atrial appendage closure device. The procedure was performed by Dr. Sanjit Jolly, an investigator in the LAAOS-4 trial, working at Hamilton Health Sciences, in Hamilton, Ontario.
“This marks an important milestone for this randomized trial, which will determine if occluding the left atrial appendage using a WATCHMAN FLX™ Left Atrial Appendage Closure Device or WATCHMAN FLX™ Pro LAAC Device can provide superior protection from stroke, on top of oral anticoagulation in patients with atrial fibrillation who are at highest risk of stroke,” says lead investigator PHRI Senior Scientist Jeff Healey, a Professor at McMaster University.
 “LAAOS-4 is prospectively evaluating a new paradigm in stroke prevention for patients with atrial fibrillation, combining mechanical and medical therapy,” adds Healey.
“LAAOS-4 is prospectively evaluating a new paradigm in stroke prevention for patients with atrial fibrillation, combining mechanical and medical therapy,” adds Healey.
Over the last 30 years, Professor Stuart Connolly, a PHRI Senior Scientist and chair of the LAAOS-4 steering committee, has led many of the key trials of anticoagulation for stroke prevention in atrial fibrillation. While chronic therapy with oral anticoagulation can prevent up to two-thirds of strokes, Connolly highlights that “between 30% and 50% of individuals stop taking this therapy within 3 years, exposing them to long-term risk.”
“Even with consistent medication use, recent research suggests that around 1 in 4 patients remain at high risk for stroke,” Connolly adds. “This underscores the pressing need for new strategies to prevent strokes in this population.”
In the landmark 2021 PHRI trial, LAAOS-III, published in The New England Journal of Medicine, the team at PHRI identified a possible solution: occluding or removing the left atrial appendage. The LAAOS-III trial demonstrated a 33% reduction in the risk of stroke with left atrial appendage removal at the time of open-heart surgery, and a 42% reduction in strokes occurring beyond 30 days post-operatively.
“The reduction in stroke with atrial appendage occlusion or removal was seen equally in patients taking and not taking anticoagulants, and this benefit got larger over the 5-6 years of study follow-up”, said Dr. Richard Whitlock, PHRI Scientist and principal investigator of LAAOS-III.
Dr. Sanjit Jolly, PHRI Scientist, an interventional cardiologist and LAAOS-4 steering committee member says, “The WATCHMAN FLX device is a safe and effective way to occlude the left atrial appendage and we are very optimistic that this device can prevent stroke in a non-surgical population to a similar extent as seen in LAAOS-III.” In the new year, the FDA-approved WATCHMAN FLX Pro Device* will be introduced in new regions and become the device used in the LAAOS-4 study.
PHRI Scientist, LAAOS-4 steering committee member and stroke neurologist Dr. Ashkan Shoamanesh says “this new paradigm of atrial appendage occlusion on top of continued anticoagulant therapy is particularly attractive for patients with AF who have already suffered a stroke.” Patients who have experienced a stroke despite being prescribed anticoagulation are at extremely high risk of another stroke, and we really have no great options to reduce their risk.”
LAAOS-4 is an investigator-initiated study, coordinated by PHRI with financial support from Boston Scientific, a global leader in developing a less invasive approach to occlude the LAA. The trial is being conducted at more than 240 centres in 12 countries, with an anticipated 4000 participants. Enrollment is expected to continue for another two years.
*The WATCHMAN FLX Pro Left Atrial Appendage Closure Device is not available for sale in all countries.