A large international study found that asundexian, a new anti‑clotting medication still under study, can reduce the risk of a second stroke in people who recently experienced a clot-related stroke or TIA (a temporary blockage of blood flow to the brain), without increasing the risk of major bleeding, a common concern with current anti‑clotting treatments.

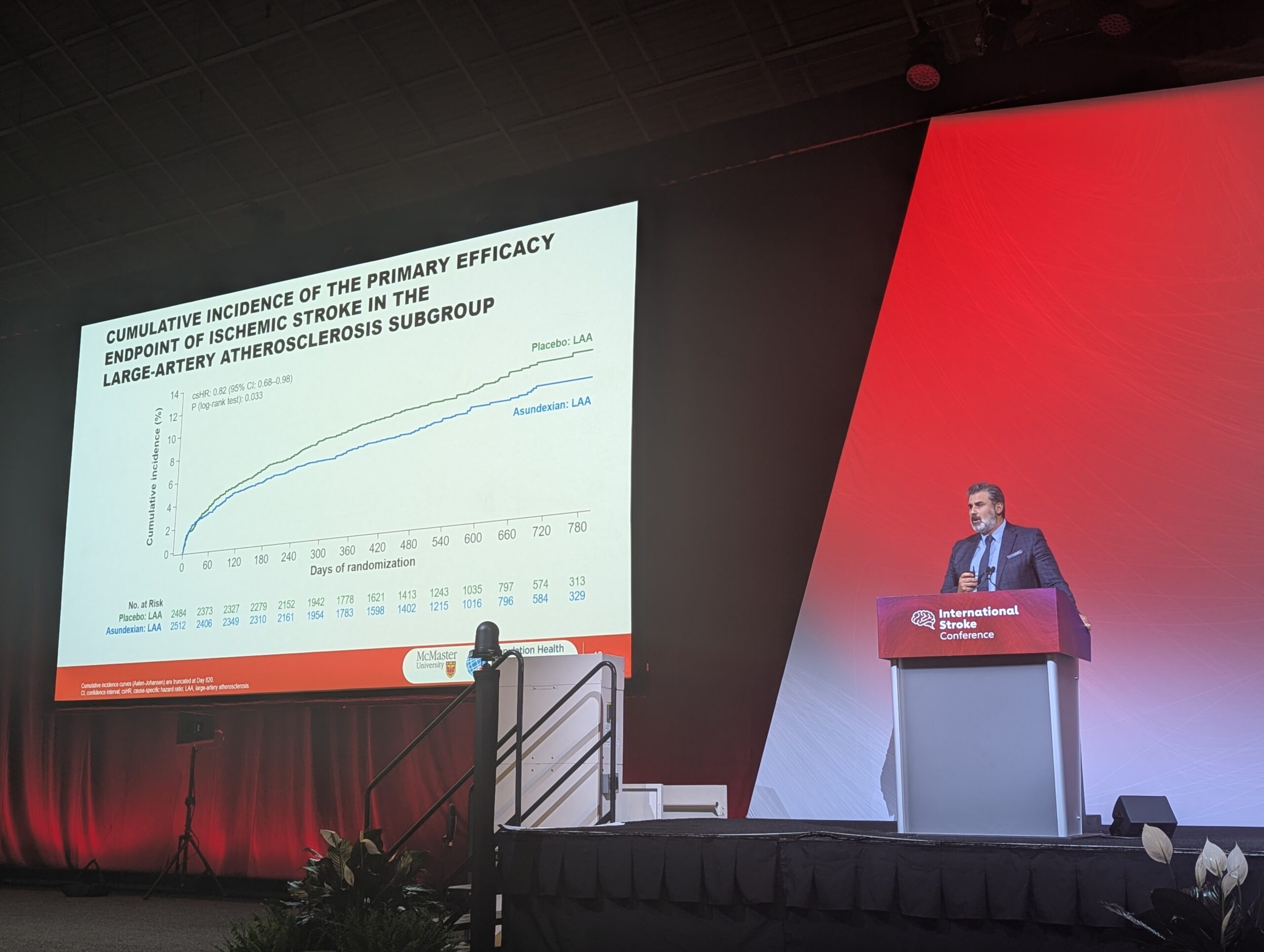
Sharma presenting at ISC 2026
“This is something researchers have been working toward for decades,” said Mike Sharma, principal investigator of the study and PHRI senior scientist. “We showed that this medication can reduce the risk of another stroke without increasing bleeding or other serious side effects, and that benefit was seen across all patients.”
Sharma presented findings from the OCEANIC‑STROKE trial at the American Stroke Association’s International Stroke Conference 2026 on February 5 in New Orleans.
The study involved 12,327 adults across 702 sites in 37 countries who were enrolled within 72 hours of a non‑cardioembolic stroke or TIA, meaning the patient did not have a condition that put them at risk of clots in the heart that could cause a stroke. Participants were randomly assigned to receive either asundexian (50 mg once daily) or a placebo, in addition to standard antiplatelet therapy such as aspirin.
Researchers found:
- 6.2 per cent of patients taking asundexian experienced another ischemic stroke (clot-related stroke), compared with 8.4 per cent of those taking placebo.
- 9.2 per cent experienced a major cardiovascular event (stroke, heart attack or cardiovascular death), compared with 11.1 per cent on placebo.
- Disabling or fatal strokes occurred in 2.1 per cent of patients taking asundexian versus 3.0 per cent in the placebo group.
- No increase in major, clinically relevant non-major, intracranial or minor bleeding was observed.
“The treatment asundexian reduced strokes by 26 per cent, and the benefit was consistent across patients of different ages, sexes, stroke severity, and stroke causes, without increasing major bleeding” added Mike.
Currently, preventing another stroke after a non‑cardioembolic stroke relies mainly on antiplatelet medications, which reduce risk only modestly and may increase bleeding when combined or used for long term.
Shoamanesh at ISC 2026
“Until now, reducing stroke risk has often been associated with higher bleeding risk. These findings give us hope for a safer way to prevent recurrent strokes,” said Ashkan Shoamanesh, co‑principal investigator of the study and PHRI senior scientist. “That’s something physicians, patients and families have been waiting for.”
Asundexian represents a new approach and works by blocking Factor XIa, a protein that contributes to harmful clot formation but plays only a small role in stopping bleeding. This allows the medication to reduce clots that can cause stroke while preserving the body’s natural ability to stop bleeding.
OCEANIC-STROKE is the first completed Phase III trial of a Factor XIa inhibitor for secondary stroke prevention. “It marks a major step toward safer and more effective long‑term treatment, that completed with remarkable scale and efficiency right here in Canada through our global research network,” said Mike.
The study was sponsored by Bayer.