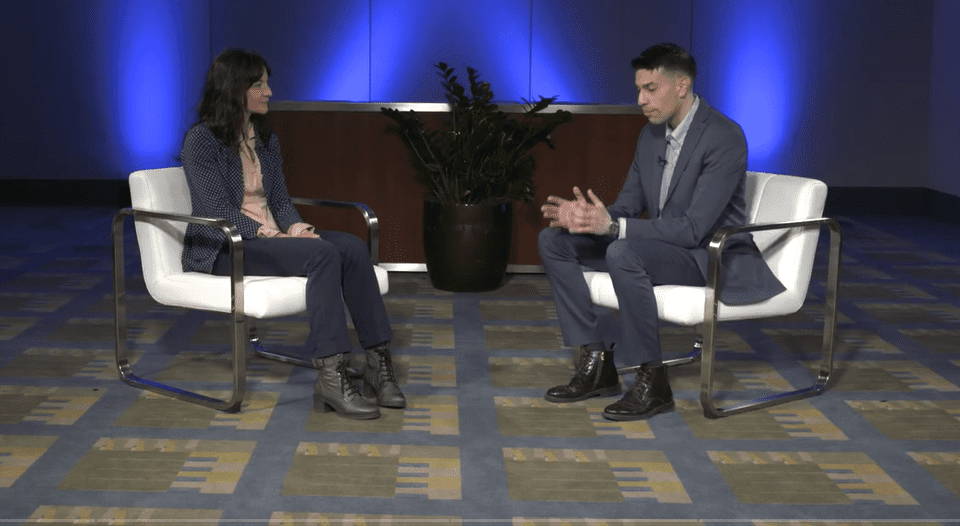Study Type
Interventional - Drug
POISE-3 was an international multicentre randomized clinical trial to assess the impact of tranexamic acid (TXA) versus placebo, and to assess the impact of managing hypotension versus standard of care (hypertension) in patients undergoing non-cardiac surgery who are at risk of a perioperative cardiovascular event.
The primary objective of POISE-3 was to determine the incidence of a composite of myocardial infarction, stroke and pulmonary embolism. Patients were followed for one year.
ACC22 - Tranexamic Acid - Download PDF ACC22 - Hypo/hypertension strategies - Download PDF POISE-3 Rationale - Download PDFInterventional - Drug
Multi-centre, blinded, randomized controlled trial
24
149
9561
2018 - 2022
PHRI


Deputy Director, Senior Scientist
Devereaux is a cardiologist, perioperative care physician, and clinical epidemiologist. He is the Director of the Division of Perioperative Care at McMaster University and a Deputy Director, Senior Scientist, and Scientific Leader of the Anesthesiology, Perioperative Medicine, and Surgical Research Group at PHRI. He is the Nominated Principal Applicant of the Accelerating Clinical Trials (ACT) Consortium, which is the Pan-Canadian Clinical Trials Consortium funded by the Canadian Institutes of Health Research (CIHR). Additionally, he holds a Tier 1 Canadian Research Chair in Perioperative Medicine.
Devereaux is a full Professor at McMaster University and the President of the Society of Perioperative Research and Care. He has published over 450 peer-reviewed papers and more than 85 book chapters, editorials, and commentaries, including 17 in the New England Journal of Medicine, 13 in the Lancet, and 11 in JAMA. He has an h-index of 115 and has given over 1000 lectures and research presentations in 41 countries. His research program focuses on major vascular complications during and after surgery, and he has led many large international randomized trials and prospective cohort studies on this topic.


Scientist
Maura Marcucci is a scientist in the Perioperative and Surgery research group at PHRI, and an Assistant Professor, Department of Medicine, and Department of Health Research Methods, Evidence, and Impact, at McMaster University. Leveraging on her background in vascular medicine, thrombosis and hemostasis disorders, and geriatrics, Maura’s current main focus of clinical research is cardiovascular and neurocognitive outcomes in noncardiac surgery. She is currently the project officer of the PeriOperative ISchemic Evaluation (POISE)-3 trial and principal investigator of the POISE-3 sub-study on delirium and cognitive decline.
She obtained her medical training and residency in Internal Medicine in Italy. In 2012-2013 she completed an MSc in Health Research Methodology and a clinical fellowship in Hematology/Medicine at McMaster University. In 2014-2016 she was an Assistant Professor at the University of Milan in Italy, before returning to Hamilton, Canada.


Senior Scientist
David Conen is currently the Principal Investigator and Senior Scientist at the Population Health Research Institute (PHRI) and an Associate Professor of Medicine at McMaster University. He serves as a staff cardiologist at St. Joseph’s Healthcare Hamilton (SJHH) and Hamilton Health Sciences (HHS) and is the Cardiology Clinical Site Lead at the Juravinski Hospital. Conen obtained his MD degree at the University of Lausanne, Switzerland, and holds a Master of Public Health degree from Harvard School of Public Health in Boston, USA. He completed a 2-year postdoctoral research fellowship in clinical epidemiology. His research focuses on perioperative medicine and atrial fibrillation.
Conen is the Principal Investigator of two large multinational clinical trials aimed at preventing and treating perioperative atrial fibrillation (COP-AF and ASPIRE-AF) and is involved in the leadership of many international cardiovascular and perioperative trials. COP-AF has recently been presented and published. His research activities also include conducting large epidemiological cohort studies that examine risk factors for the occurrence and consequences of atrial fibrillation. He has published over 250 manuscripts in peer-reviewed scientific journals and has received several prestigious grants and awards in both Switzerland and Canada. Conen has extensive knowledge in the design, development, management, analysis, interpretation, and reporting of clinical trials and large-scale epidemiology studies.


Scientist
Andre Lamy is a cardiac surgeon practicing at the Hamilton Health Sciences since 1996, and Professor in the Department of Surgery, McMaster University. He led the Canadian Institute of Health Research funded CORONARY trial, which evaluated off-pump CABG surgery versus on-pump CABG surgery in 4752 patients. The results were published in the New England Journal of Medicine in 2012 and 2013.


Scientist
Flavia Kessler Borges is a Scientist at the Population Health Research Institute and an Internist and Assistant Professor in the Department of Medicine and the Perioperative Care Division at McMaster University. She undertook her Masters in Health Sciences and her PhD in Cardiovascular Sciences in Brazil. She undertook a post doctorate and a clinical Perioperative Vascular fellowship under the direction of PHRI Senior Scientist PJ Devereaux at McMaster University. Her research is focused on perioperative cardiac biomarkers, and perioperative strategies to improve patient important outcomes in surgical patients, specially in hip fracture patients. She holds an Internal Career Research Award from Hamilton Health Sciences, a CIHR and NIH large grants as the Principal Investigators of the HIP ATTACK-2 trial.
Scientist
Sandra Ofori is a Scientist in the Perioperative and Surgery research group at PHRI, an Assistant Professor in McMaster University’s Department of Medicine (cardiology), and a general cardiologist in Hamilton Health Sciences and St Joseph’s hospital, Hamilton. Her research interests are in the areas of perioperative care and cardiovascular disease prevention.
Her current research program is focused on optimizing the health of patients undergoing surgery with a focus on perioperative smoking cessation and secondary prevention strategies. Ofori is actively involved in the conduct of large international perioperative clinical trials coordinated from PHRI and was the project officer of the Post Discharge after Surgery Virtual Care with Remote Automated Monitoring Technology (PVC-RAM) 1 and 3 trials and a Co-Investigator in the PVC-RAM-2 trial. She is leading the smoking cessation program in the perioperative care group and is the Co-PI of the PREVENT trial.
Ofori completed her residency training in Internal Medicine and Cardiology at the University of Port Harcourt, Nigeria. She holds a Master’s degree in Preventive Cardiology from the Imperial College, London UK, and completed a Perioperative Medicine fellowship and a PhD in Health Research Methodology (Clinical Epidemiology) from McMaster University both under the supervision of PHRI Senior Scientist PJ Devereaux. She was in the third cohort of the World Heart Federation Salim Yusuf Emerging Leaders Program that was focused on the reduction of the global burden of hypertension


Investigator
Michael Ke Wang is an Investigator at PHRI and an MSc student in the Health Research Methodology Program at McMaster University, supervised by David Conen. His main interests are in perioperative medicine and perioperative atrial fibrillation. Michael is actively involved in the conduct of large international perioperative clinical trials coordinated from PHRI; he is the project officer of the COP-AF and ASPIRE-AF trials.
Michael is a Clinical Scholar in the Division of General Internal Medicine at McMaster University. He obtained his medical degree from the University of Ottawa and completed his internal medicine residency, general internal medicine fellowship, and perioperative vascular training at McMaster University.


Investigator
Pavel Roshanov, an internal medicine physician and nephrologist, is completing a fellowship in kidney transplantation at McMaster University. He has published more than 50 papers in the areas of perioperative care and medical informatics.


Director, Statistics; Senior Scientist
Shrikant Bangdiwala, PhD, has extensive experience in the design, conduct and analysis of multi-center observational and experimental studies, having worked on clinical and community-based randomized controlled trials in congestive heart failure, cardiovascular risk factors, functional bowel disease, and obesity prevention. His statistical research interests include non-parametric methods, methodology for clinical trials, reliability and validity of diagnostic tests, and graphical methods for descriptive analyses.
A Professor in McMaster University’s Department of Health Research Methods, Evidence and Impact, he is a former Fulbright senior specialist in global public health, and holds faculty positions in universities in Chile, South Africa and India.
He is a member of the USA NIH National Institute of Allergy and Infectious Diseases (NIAID) “COVID-19 Preventive mAb Data and Safety Monitoring Board” (DSMB) that will be reviewing and monitoring the US government-supported clinical trials of candidate preventive monoclonal Antibodies for SARS-CoV-2 and COVID-19. He also chairs the Multinational DSMB for the division of AIDS at NIH, and is a member of the Gastrointestinal Drugs Advisory Committee of the FDA.


Senior Scientist
Richard Whitlock is Associate Chair, Research, and a Professor at the Department of Surgery, McMaster University. He was awarded the inaugural Canada Research Chair in Cardiovascular Surgery in 2020.
As well as being a PHRI Scientist, Richard is a cardiac surgeon and intensive care physician at Hamilton Health Sciences. His clinical focus is on aortic valve intervention and aortic surgery. He is a lead investigator for the CIHR funded studies SIRS, LAAOS III, and TRICS III, which have established a network of more than 120 centres to address important questions in his field.
He has published more than 90 articles in referred journals. Medically qualified at the University of Toronto, Richard received his specialist training in cardiac surgery and critical care medicine at McMaster University. In 2012, he received his PhD in clinical epidemiology.


Senior Scientist
John Eikelboom is Professor, Department of Medicine, McMaster University, Senior Scientist, Population Health Research Institute, Hamilton Health Sciences Corporation, and Hematologist, Thrombosis Service, Hamilton General Hospital. He completed training in Internal Medicine and Hematology in Perth, Australia, in 1998 and in Health Research Methodology at McMaster University, Canada, in 2000.
He has authored or co-authored more than 800 articles in peer-reviewed journals and for the past decade has been listed annually by the Web of Science among the top 1% of cited researchers. He holds the Jack Hirsh/Population Health Research Institute Chair in Thrombosis and Atherosclerosis. His current research, supported by peer reviewed funding from the Canadian Institutes for Health Research and the Gates Foundation focuses on the efficacy and safety of antithrombotic therapies in arterial, venous, cardiac, and procedure-associated thromboembolism as well as strategies to reduce the burden of the “big three” infectious diseases (HIV, TB, malaria) on the African continent.


Operations Manager, Statistics
Kumar Balasubramanian has been an integral part of the PHRI Statistics department since November 2013 when he joined as a Statistical Analyst; then became Statistical Analyst II in 2016, and Statistical Coordinator in 2018. In August 2021 he became the department’s Statistical Operations Manager.
Before coming to PHRI, Kumar held academic statistical positions at University of Calgary and University of Toronto. He holds a Masters in Biostatistics from University of Toronto.


Founder and Emeritus Executive Director, Senior Scientist
Salim Yusuf is an internationally renowned cardiologist and epidemiologist, whose work over 40 years has substantially influenced prevention and treatment of cardiovascular disease. Medically qualified from St John’s Medical College in Bangalore in 1976, he received a Rhodes Scholar-ship and obtained a DPhil from Oxford, during which he (along with Richard Peto, Rory Collins and Peter Sleight) initiated the concepts of large, simple trials, and meta-analysis. He coordinated the first ISIS trial (which established the structure for future international collaborative work in cardiovascular and several other diseases) that demonstrated the value of beta-blockers in myo-cardial infarction, and was a member of steering committees for all subsequent ISIS trials.
In 1984, following clinical training in medicine and cardiology in the UK, he moved to the National Institutes of Health, Bethesda, USA, where he led the SOLVD trial (establishing the value of ACE-inhibitors on LV dysfunction) and DIG trial (clarifying the role of digitalis).
In 1992 he moved to McMaster University as head of cardiology, where he established an inter-national program of research in cardiovascular diseases and prevention, culminating in the creation of the Population Health Research Institute. His therapeutic trials have established the roles of ACE-inhibitors in CVD prevention, dual antiplatelet therapies in acute coronary syndromes, novel antithrombotic therapies, and most recently the value of the polypill in substantially pre-venting heart attacks and strokes globally, and at low cost. The Polypill was recently included by the WHO in its Essential Medicines List.
His epidemiologic work in over 80 countries involving all inhabited continents of the world shows the majority of risks of both heart attacks and strokes are attributable to a few risk factors. He currently leads the PURE study exploring the role of societal and environmental factors in CVD. This study (PURE) involves 200,000 people from over 800 communities in 27 high, middle and low-income countries.
He has built capacity for clinical and population research across Canada and the world by establishing research networks involving over 1500 sites in 102 countries. He has trained over 100 researchers, many of whom are now nationally or internationally renowned leaders in medical research. He has helped develop major research institutes or programs in Canada, India, Argentina, Brazil, S. Africa, Saudi Arabia, Malaysia, and China.
He holds a Heart and Stroke Foundation of Ontario Research Chair, was a Senior Scientist of the Canadian Institutes of Health Research (1999-2004), and has received (among over 100) the Lifetime Research Achievement award of the Canadian Cardiovascular Society and the World Heart Federation, the Paul Wood Silver Medal of the British Cardiac Society, the European So-ciety of Cardiology gold medal, the American Heart Association Clinical Research Award, the Killam Prize, and the Canada Gairdner Wightman Award in 2014. He has been inducted into the Royal Society of Canada, been appointed an Officer in the Order of Canada, and has been in-ducted into the Canadian Medical Hall of Fame in 2014. In 2023 he was elected as an Honorary Fellow of St. John’s College, Oxford. He has been conferred 4 honorary doctorates.
He has published over 1400 articles, and was the second most cited researcher in the world for 2011, and has been among the highest cited scientists in the world (his H index is currently 17th of all scientists in history) for over a decade. He is Past President of the World Heart Federation, where he initiated the Emerging Leaders program (now named after him) to build capacity for research in all continents of the world, with the aim of halving the CVD burden globally within a generation. This program has already trained over 250 individuals from 50 countries.


Associate Deputy Director, Scientist
Emilie Belley-Côté is an Assistant Professor in the Department of Medicine at McMaster University; she practices critical care cardiology in the cardiovascular intensive care unit and coronary care unit at the Hamilton General Hospital. Her research interests include perioperative cardiac surgery care, knowledge synthesis and guideline development. At this stage in her career, she has more than 120 publications, including articles in NEJM, Lancet, JAMA, as well as first-tier critical care journals.
She obtained her MD from Université de Sherbrooke in 2006. After internal medicine and cardiology training, as well as an MSc in Clinical Sciences, she completed a critical care fellowship at McMaster. In 2019, she completed a PhD in Health Research Methodology at McMaster University.


Scientist
William is a cardiologist and a native of Fredericton, New Brunswick, Canada. He completed Medical School and Internal Medicine Residency at Queen’s University and Adult Cardiology Residency at the University of Manitoba. He completed a Fellowship in Cardiac Electrophysiology and Pacing and a PhD in Health Research Methodology at McMaster University. He is the only Canadian to receive the European Heart Rhythm Association’s Diploma of Advanced Studies in Cardiac Arrhythmia Management.
He is a consultant cardiologist and arrhythmia service lead at St. Joseph’s Healthcare Hamilton. He leads the post-operative atrial fibrillation program at Hamilton Health Sciences.
William is a Scientist at PHRI and an Assistant Professor at McMaster University. He holds research funding from Canadian Institutes of Health Research (CIHR), the Heart and Stroke Foundation of the Canadian Stroke Prevention Intervention Network (C-SPIN), the Canadian Cardiovascular Society (CCS) Atrial Fibrillation Awards Program and the Heart and Stroke Foundation of Canada.
He has published more than 150 peer-reviewed articles, including first author works in JAMA, Annals of Internal Medicine, Circulation and the European Heart Journal. He was the 2023 recipient of the Canadian Cardiovascular Society’s Young Investigator Award. His research interests include atrial fibrillation screening, medical therapy of atrial fibrillation and the treatment of new-onset atrial fibrillation in cardiac surgery patients.


Associate Scientist
Emmanuelle Duceppe is a principal scientist with Centre hospitalier de l’Université de Montréal (CHUM) Research Centre in Montreal, Quebec. She is also an internist and clinical assistant professor, Department of Medicine, Université de Montréal.
Her research interests include: prediction of preoperative risk in non-cardiac surgery; perioperative interventions and clinical outcomes in non-cardiac surgery; biomarkers for prediction and early identification of perioperative complications; pre- and postoperative management of patients in day surgery; statistical methodology for predictive model development; and cohort studies and clinical trials.


Associate Scientist
Amit Garg is the Associate Dean, Clinical Research, at the Schulich School of Medicine & Dentistry, Western University, has practiced as a staff nephrologist at the London Health Sciences Centre in London, Ontario, Canada since 2003, and is a Professor of Medicine, Epidemiology and Biostatistics at Western University, with a cross-appointment in the Department of Health Research Methods, Evidence, and Impact (HEI) at McMaster University. A past president of the Canadian Society of Nephrology, Amit Garg serves as the current Ontario Lead of the Kidney, Dialysis and Transplantation Program at ICES.
He values his ongoing collaborations with PJ Devereaux and other leading clinician-scientists at PHRI, which has led to several sub-studies funded by the Canadian Institutes of Health Research which examined the effects of perioperative interventions on the risk of acute kidney injury (off-pump cardiopulmonary bypass surgery) in the CORONARY study, and more. A current interest is in pragmatic randomized trials embedded into routine healthcare delivery.


Scientist
Michael McGillion is Associate Professor, and Assistant Dean, Research, at the School of Nursing, McMaster University. He is the Heart and Stroke Foundation/Michael G. DeGroote Endowed Chair in Cardiovascular Nursing Research, and the International Visiting Professor of Digital Health, at Coventry University in the UK.
He is an internationally-recognized researcher in the area of persistent forms of cardiac pain such as refractory angina and unrelieved chest pain following successful revascularization procedures. He was Chair of the Joint Canadian Cardiovascular Society – Canadian Pain Society guidelines for the management of refractory angina, funded by the Canadian Institutes of Health Research (CIHR). He is Principal Investigator of the largest CIHR-funded, international prospective cohort study to examine social and psychological predictors of chronic post-surgical pain following cardiac surgery. His research focuses on remote automated monitoring and virtual recovery support for people recovering from cardiac and vascular surgery, decision support for people living with RFA, and global-scale, web-based dissemination of new evidence on persistent forms of cardiac pain.
Mike has been recognized for his research and advocacy by receiving the Canadian Pain Society Early Career Award and the McMaster University Arch Award for outstanding contributions to society; and was the first University Scholar (2019) from the McMaster School of Nursing.


Head of Operations
Jessica Vincent has more than 15 years’ experience in coordinating and managing large, international clinical trials. She holds an Honours Bachelor of Science Degree from Queens University, and a Master of Clinical Epidemiology Degree from the University of Newcastle.


Research Project Manager II
Ingrid Copland has been working in research for McMaster University since 1991. She has been at PHRI for 23 years where she has coordinated large, international, industry and investigator initiated trials. Since 2014 she has been with the perioperative and surgery department coordinating observational, drug and device trials.
These organizations were involved in this study as: grant providers; funders (including in-kind); national leaders not directly involved in the research team, and other supporters.
Research highlights, events, media coverage of PHRI and more.
April 4, 2022
PHRI Scientist Maura Marcucci presented today at ACC 2022 on the arm of the POISE-3 trial looking at blood pressure...
Read MoreApril 4, 2022
Banner image © ACC/Nick Agro 2022 Day 1 (April 2) at ACC 2022 was a big day for PHRI researchers....
Read MoreApril 2, 2022
Event photos courtesy ©ACC/Nick Agro 2022 The large, international trial, POISE-3, has found a drug that helps the blood to...
Read MoreData will be shared as per the PHRI Data Sharing Policy, which requires approval of the proposed use of the data by a review committee.
We wanted to undertake a large international factorial RCT to establish the effects of rosuvastatin...
The objective of the POISE-2 study was to determine the impact of clonidine versus placebo...
The objective of the POISE study was to evaluate the effects of metoprolol (a beta-blocker...
Back To Top
Don't miss out on the latest health research news from the Population Health Research Institute in Hamilton, Canada